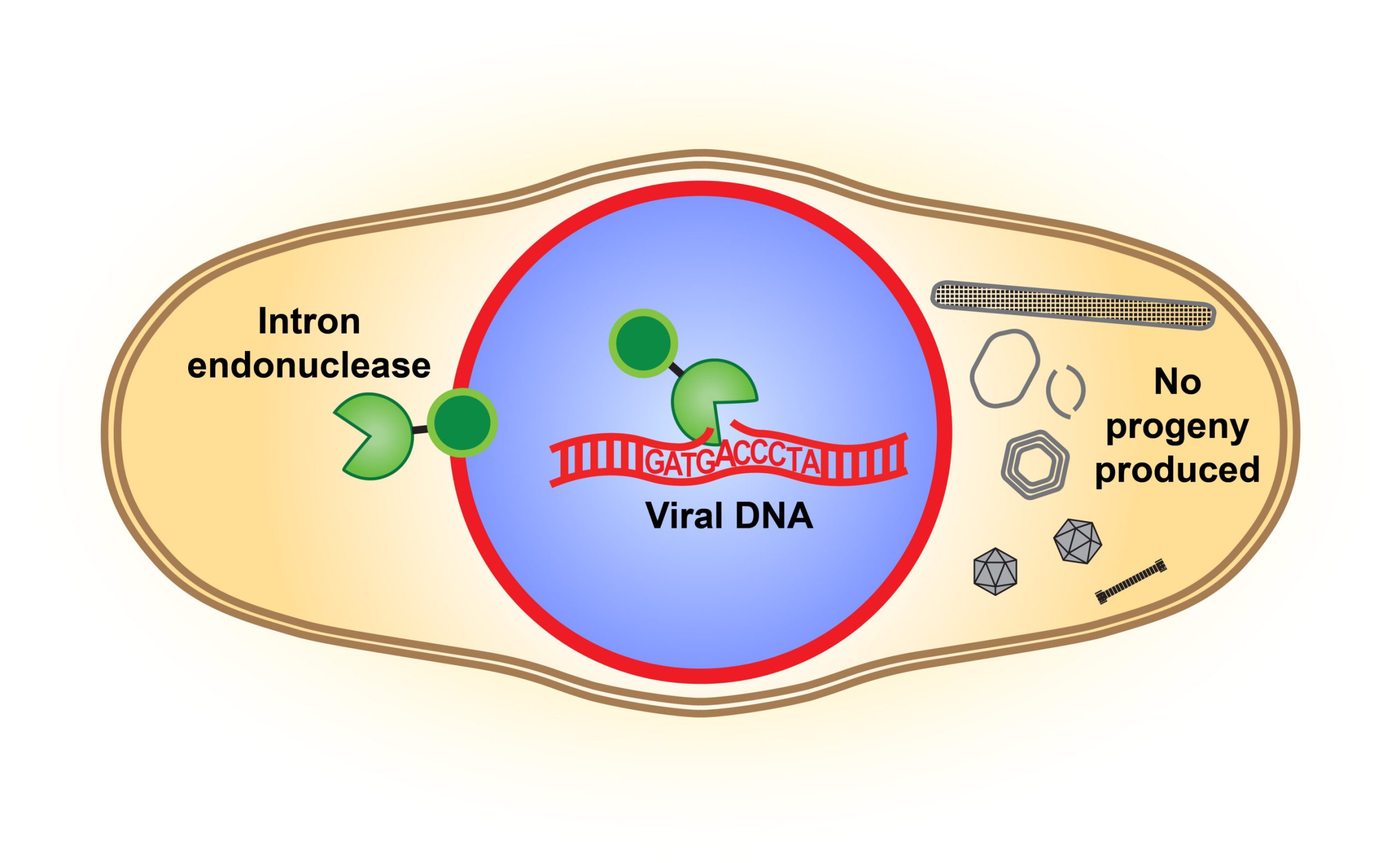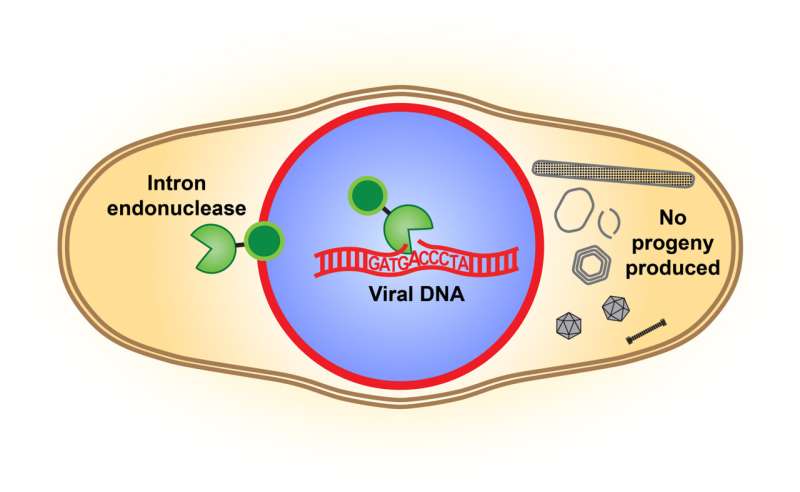
An illustration of the intron endonuclease cutting the DNA of a competing virus and disrupting its reproduction. Credit: Pogliano Labs, UC San Diego
Strange bits of DNA hidden in the genomes of all living things have historically been ignored because they seemed to play no role in the fight for survival, researchers thought.
These bits of DNA became known as “selfish genetic elements” because, as far as scientists could tell, they existed to reproduce and propagate themselves, without any benefit to their host organisms. They were seen as genetic freeloaders, passed insignificantly from one generation to the next.
Research conducted by scientists at the University of California San Diego has provided new evidence that such DNA elements may not be so selfish after all. Instead, they now appear to be a significant factor in the dynamics between competing organisms.
Publish in the magazine ScienceResearchers from the School of Biological Sciences studied selfish genetic elements in bacteriophages (phages), viruses that are considered the most common organisms on Earth. To their surprise, researchers discovered that selfish genetic elements, known as “mobile introns”, give their virus hosts a distinct advantage when competing with other viruses: phages have used mobile introns as a weapon to disrupt the ability of competing phage viruses to reproduce.
“This is the first time that a selfish genetic element has been shown to provide a competitive advantage to the host organism it has invaded,” said Erica Birkholz, co-first author of the study and a postdoctoral researcher in the Department of Molecular Biology. “Realizing that selfish genetic elements are not always purely ‘selfish’ has major implications for understanding the evolution of genomes across all kingdoms of life.”
Decades ago, biologists noted the existence of selfish genetic elements but were unable to characterize any role they play in helping the host organism survive and reproduce. In the new study, which focused on examining “jumbo” phages, the researchers analyzed the dynamics as two phages co-infected a single bacterial cell and competed with each other.
They looked closely at the endonuclease, an enzyme that serves as a DNA-cutting tool. The endonuclease of a phage’s mobile intron, the studies showed, disrupts the genome of the competing phage. Therefore, the endonuclease is now seen as a combat tool, as it has been documented to cut an essential gene in the competing phage’s genome. This sabotages the competitor’s ability to properly assemble its own offspring and reproduce.
“This armed intron endonuclease gives a competitive advantage to the phage that carries it,” Birkholz said.
According to the researchers, the finding is particularly important in the evolutionary arms race between viruses because of the constant competition for co-infections.
“We were able to really delineate the mechanism that gives an advantage and how that happens at the molecular level,” said Chase Morgan, a doctoral student in biological sciences and co-first author of the paper. “This incompatibility between selfish genetic elements becomes molecular warfare.”
The study’s findings are important because phage viruses are emerging as therapeutic tools in the fight against antibiotic-resistant bacteria. As doctors deploy “cocktails” of phages to combat infections in this growing crisis, the new information will likely be useful when multiple phages are deployed. Knowing that certain phages use selfish genetic elements as weapons against other phages could help researchers understand why certain combinations of phages may not reach their full therapeutic potential.
“The phages in this study could be used to treat patients with bacterial infections associated with cystic fibrosis,” said Biological Sciences Professor Joe Pogliano. “Understanding how they compete with each other will allow us to make better cocktails for phage therapy.”
The authors of the article are: Erica Birkholz, Chase Morgan, Thomas Laughlin, Rebecca Lau, Amy Prichard, Sahana Rangarajan, Gabrielle Meza, Jina Lee, Emily Armbruster, Sergey Suslov, Kit Pogliano, Justin Meyer, Elizabeth Villa, Kevin Corbett, and Joe Pogliano.
More information:
Erica A. Birkholz et al, An intronic endonuclease facilitates interference competition between co-infecting viruses, Science (2024). DOI: 10.1126/science.adl1356. www.science.org/doi/10.1126/science.adl1356
Offered by University of California – San Diego
Quote: Phage viruses used to treat antibiotic resistance gain advantage by cutting off competitors’ ability to reproduce (2024, July 4) Retrieved July 5, 2024, from https://phys.org/news/2024-07-phage-viruses-antibiotic-resistance-gain.html
This document is subject to copyright. Except for fair dealing for private study or research, no part may be reproduced without written permission. The contents are supplied for information purposes only.